HepaFat for Patients
Frequently Asked Questions
Please click on the question(s) below to expand the answer for each. If your question is not listed below, please contact us directly at [email protected] for further information.
- Simple fatty liver, also called non-alcoholic fatty liver (NAFL), is a form of NAFLD in which you have fat in your liver but little or no inflammation or liver cell damage. Simple fatty liver typically does not progress to cause liver damage or complications;
- Non-alcoholic steatohepatitis (NASH), occurs when inflammation is present in the liver with evidence of liver cell damage, as well as fat. Inflammation and liver cell damage can trigger fibrosis, or scarring of the liver. NASH may also lead to cirrhosis or liver cancer.
The HepaFat-Scan software is the first and only method with regulatory clearances (USA – FDA, Europe – CE Mark, Australia – TGA) for quantitative measurement of liver fat that has been clinically validated against independent measurements of the volume fraction of fat in liver biopsy specimens in a clinical study.
HepaFat-Scan reports volumetric fraction of fat in the liver, a measure that can be directly compared to a volumetric biopsy fat fraction measurement.
HepaFat-Scan is now available for routine clinical use and for use in pharmaceutical company clinical trials.
When interpreted by a trained physician, the results provide information that can aid in diagnosis.
HepaFat-Scan has the following regulatory clearances:
- In the USA by the FDA (Food and Drug Administration);
- In Australia by the TGA (Therapeutic Goods Administration);
- In Europe (CE Mark)
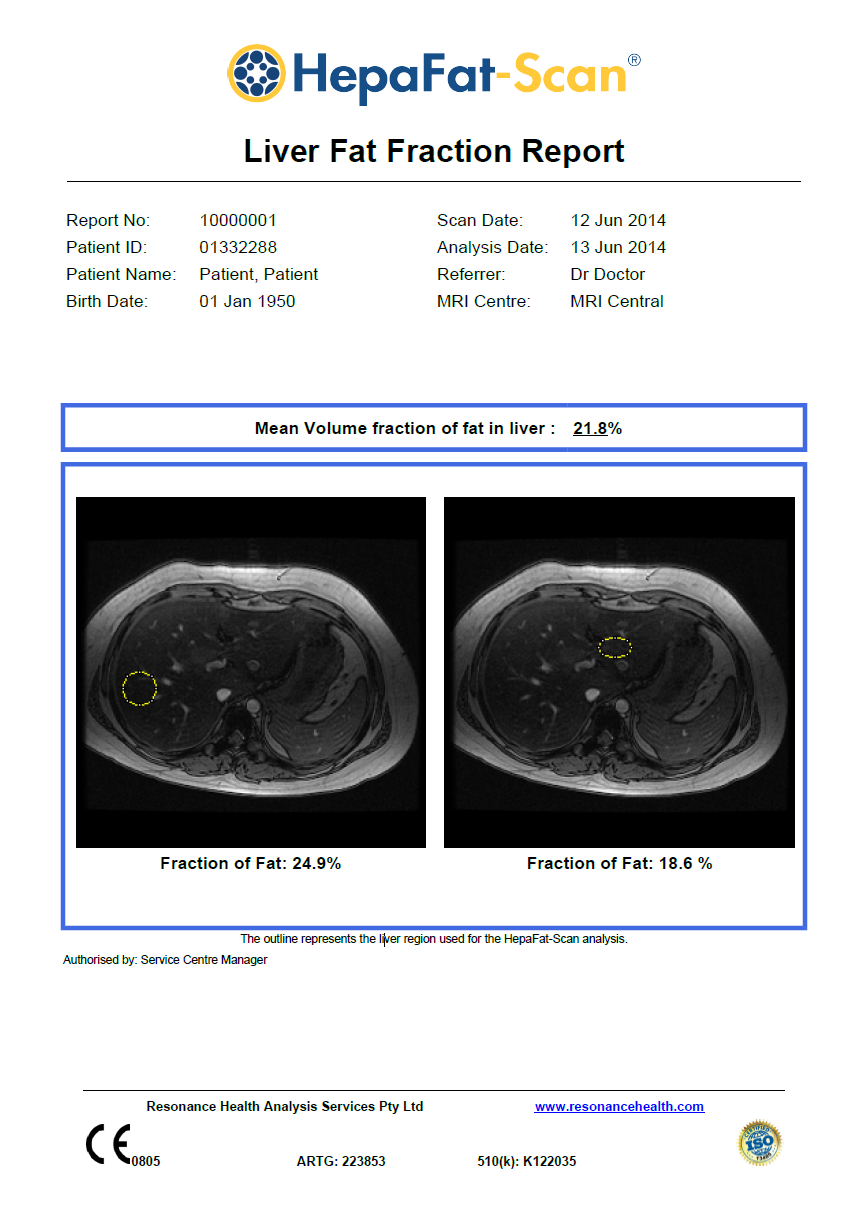
- Patient information can be seen on the first page at the top of the report.
- On the front page, the mean volume fraction of fat in the liver is displayed in the square box below the patient information. In this report, the 21.8% percentage indicates the mean volume fraction of fat in the liver of the patient.
HepaFat-AI is a fully automated analysis service that uses Artificial Intelligence (AI) combined with a non-invasive MRI-based technique to automatically analyse MRI datasets to generate an estimate of the patient’s volumetric liver fat fraction (VLFF).
HepaFat-AI has regulatory clearances from the US FDA, Australian TGA, and European CE marking.
No. HepaFat-Scan and HepaFat-AI use images acquired using magnetic resonance imaging (MRI). MRI works using electro-magnetic energy, not ionizing radiation. There are some restrictions associated with MRI use, such as in pregnancy or in people who have metal implants, but these will be discussed with patients by the MRI technologists beforehand.
HepaFat-Scan and HepaFat-AI use MRI images that are acquired using single breath hold techniques. Results are then analysed and returned instantly back to the clinician (HepaFat-AI) or to the MRI Centre within a target time of two business days (HepaFat-Scan).
Sites that are verified (set up) for HepaFat-Scan or HepaFat-AI should be able to assist you with information on pricing for these services.